Aplicación de la neurocirugía endoscópica en el tratamiento de las hemorragias hipertensivas en los ganglios basales
Introducción. La relevancia de la neurocirugía endoscópica en el tratamiento de las hemorragias hipertensivas de los ganglios basales no se conoce en buena medida.
Objetivo. Comparar la eficacia clínica de la neurocirugía endoscópica mínimamente invasiva con la de la microcirugía con craneotomía de ventana pequeña (SBWC) en el tratamiento de las hemorragias hipertensivas de los ganglios basales.
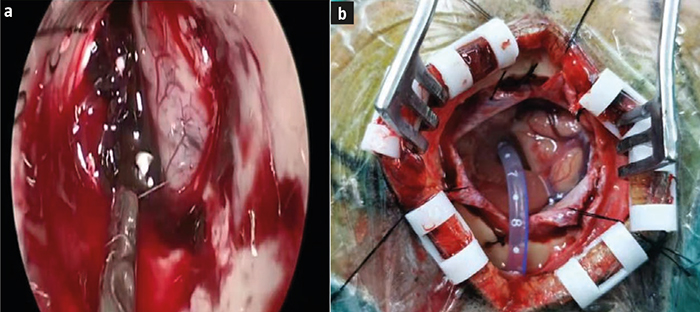
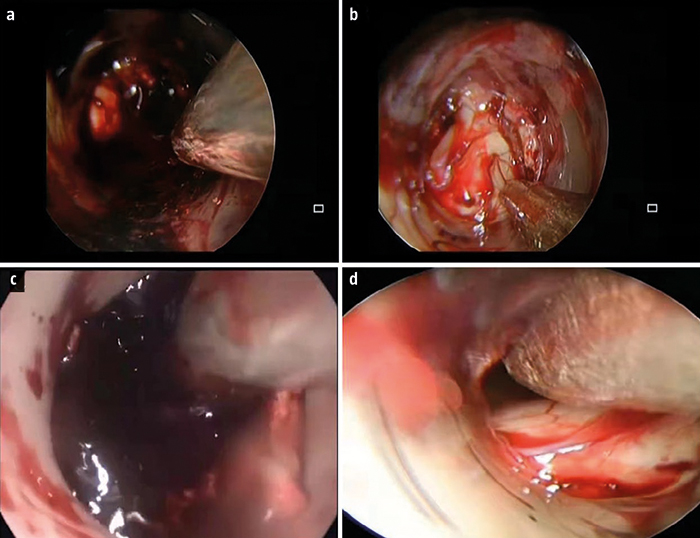
Pacientes y métodos. Análisis retrospectivo de los datos clínicos de 174 pacientes con hemorragia hipertensiva de los ganglios basales tratados en nuestro hospital desde enero de 2018 hasta septiembre de 2020. Los pacientes se dividieron en dos grupos: uno sometido a neurocirugía endoscópica mínimamente invasiva (n = 90) y otro a microcirugía con SBWC (n = 84). Se compararon la duración de la operación, la tasa de eliminación del hematoma, la recidiva hemorrágica y el pronóstico.
Resultados. En el grupo sometido a la endoscopia mínimamente invasiva, tanto la duración de la intervención como el tiempo de hemostasia fueron significativamente más breves, y la pérdida de sangre durante la intervención fue significativamente menor que en el grupo de microcirugía con SBWC (p < 0,001). La puntuación preoperatoria de la escala de coma de Glasgow (GCS) era de 8,64 ± 1,04 puntos en el grupo de la endoscopia y de 8,68 ± 1,02 puntos en el de la microcirugía (p > 0,05). A las 24 horas de la intervención, la puntuación de la GCS en los sometidos a la neuroendoscopia aumentó hasta 12,89 ± 1,56, con una diferencia significativa respecto al grupo de la microcirugía, que presentaba 11,18 ± 1,14 puntos (p < 0,001). El volumen del edema cerebral fue de 11,82 ± 3,25 mL en el grupo de la neuroendoscopia mínimamente invasiva y de 18,89 ± 3,15 mL en el de la microcirugía (p < 0,001). En comparación con el grupo sometido a esta última, en el grupo de la endoscopia, la eliminación del hematoma fue más extensa y el pronóstico resultó más favorable.
Conclusiones. La neurocirugía endoscópica mínimamente invasiva se mostró más eficaz que la microcirugía con SBWC en el tratamiento de las hemorragias hipertensivas de los ganglios basales.
Palabras clave. Cirugía mínimamente invasiva. Craneotomía. Hemorragia hipertensiva de los ganglios basales. Microcirugía. Neurocirugía endoscópica. Ventana ósea pequeña.
|
 Castellano
Castellano
 English
English